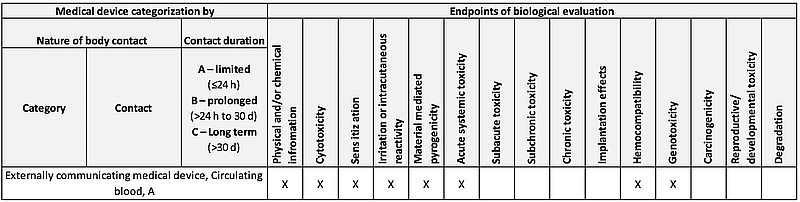
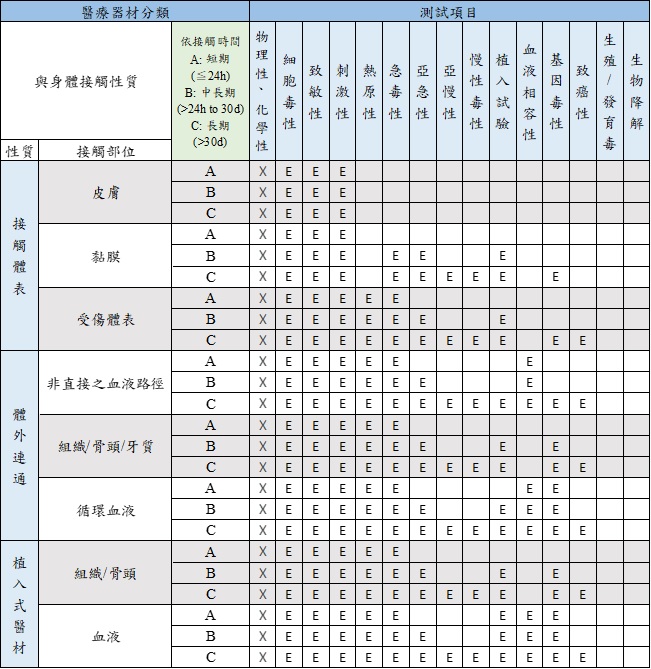
Understanding Food-Grade Vs. Biocompatibility For Medical Device Materials | Medical Product Outsourcing

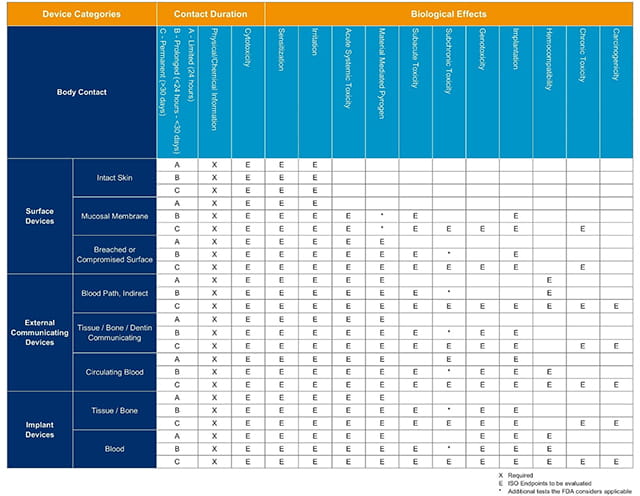
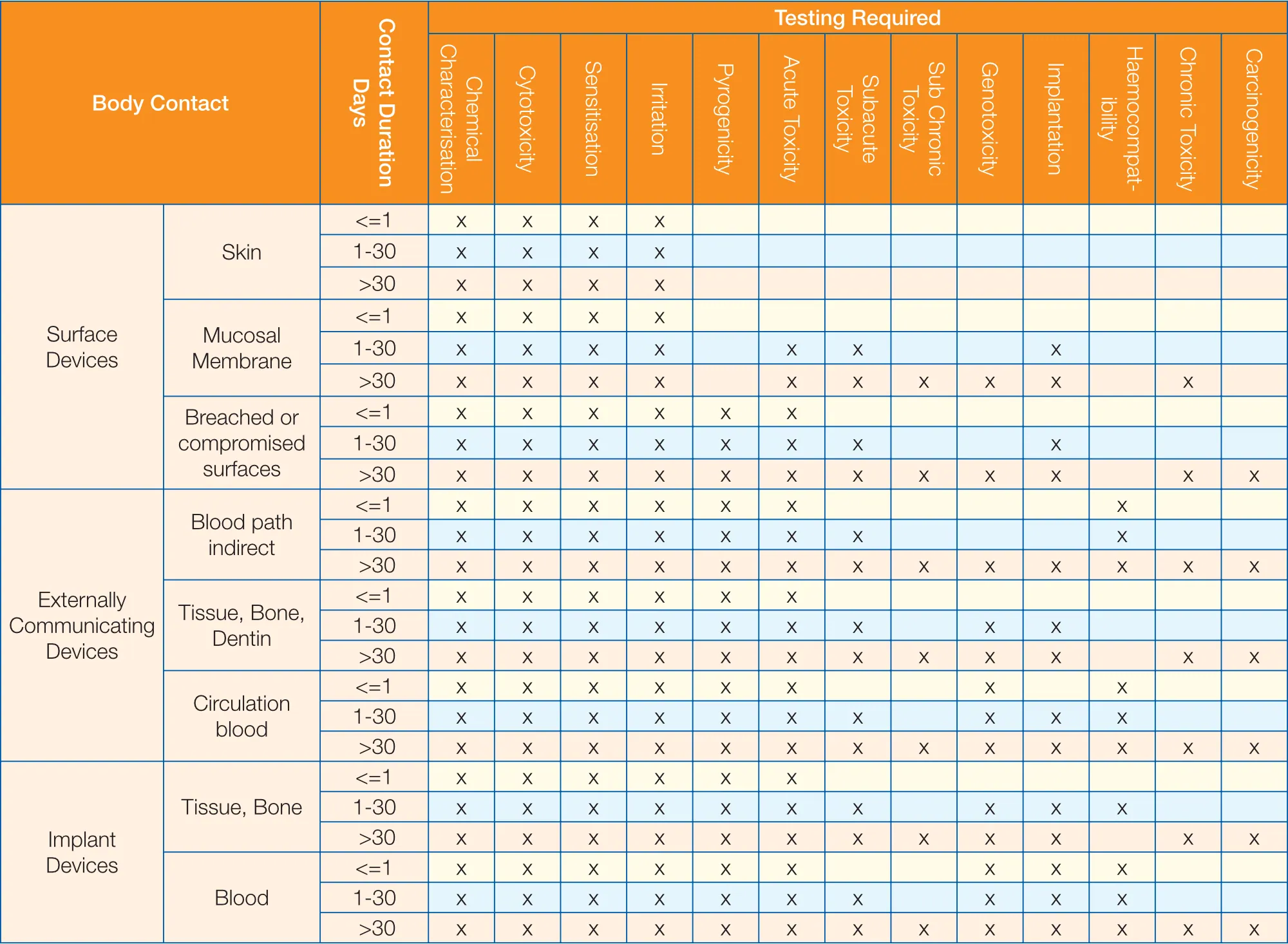
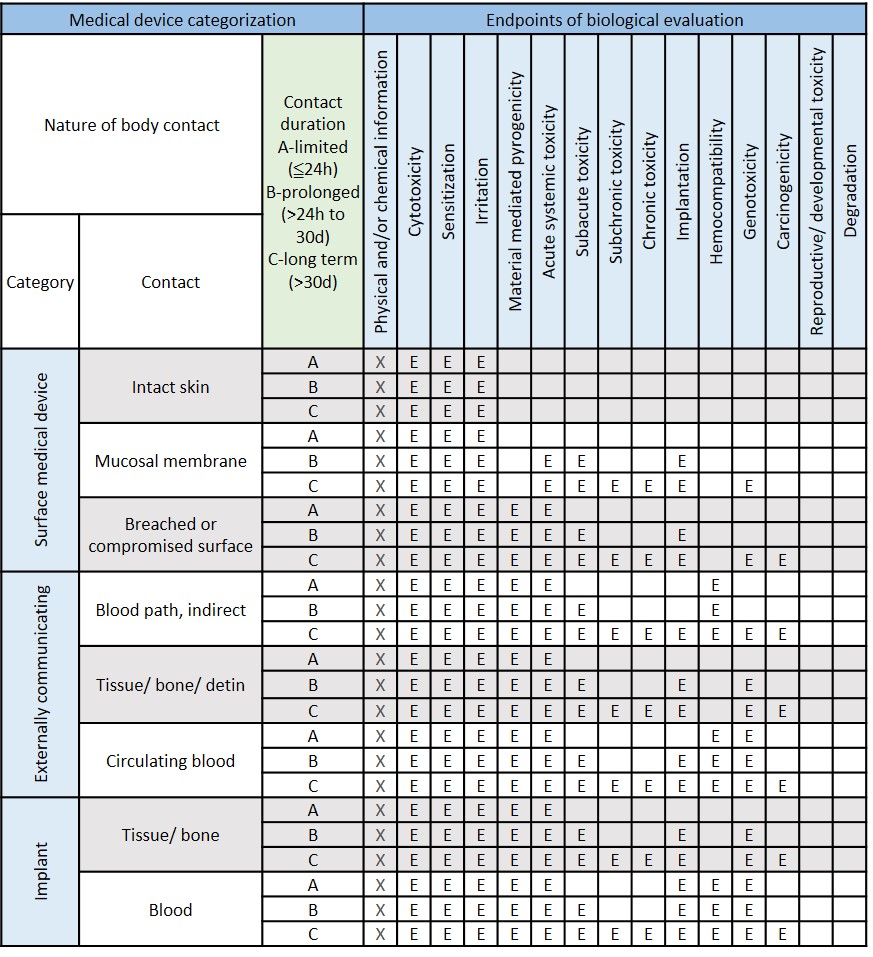
File:Initial Consideration from ISO- 10993, Biological Evaluation of Medical Devices.jpeg - Wikimedia Commons
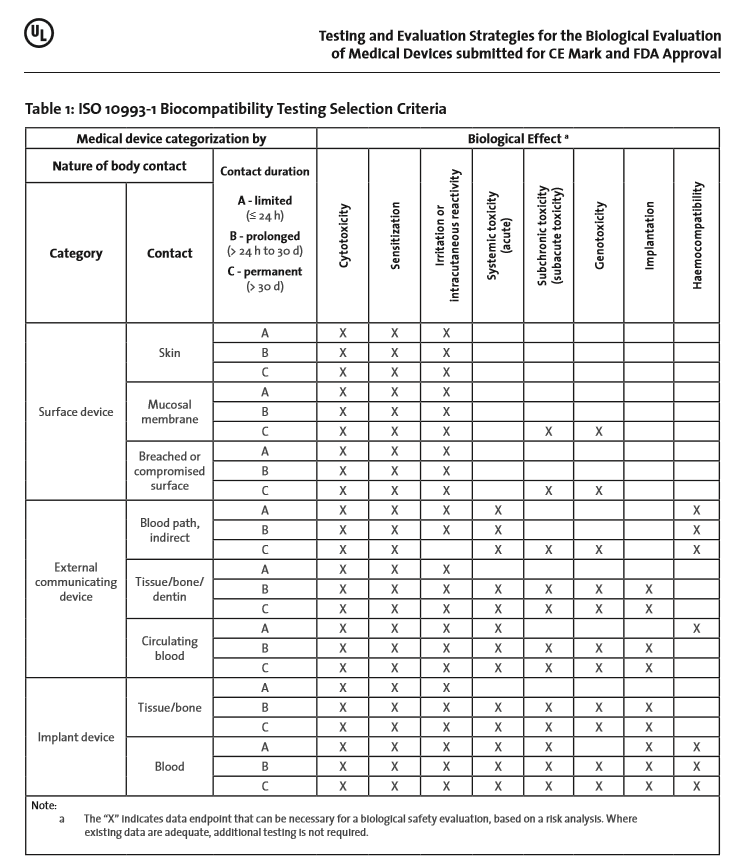
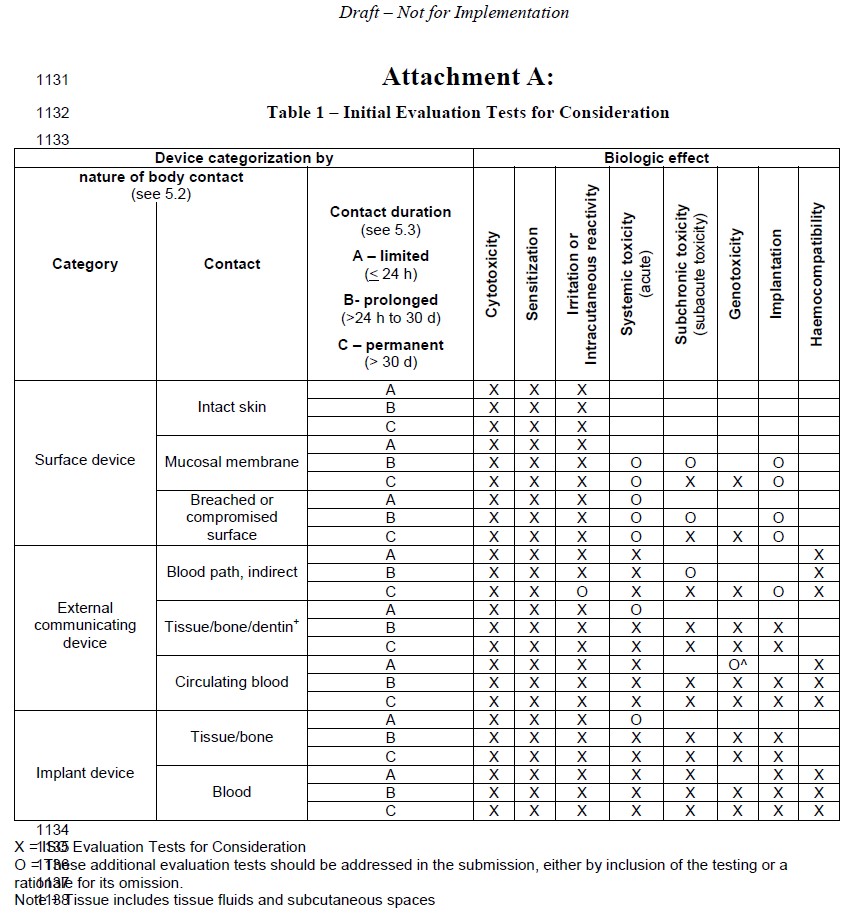
Biological Compatibility of Medical Service (ISO 10993) - Superlab CRO -health food, medical device, chemicals, pharmaceutical research (GLP&TAF accredited)

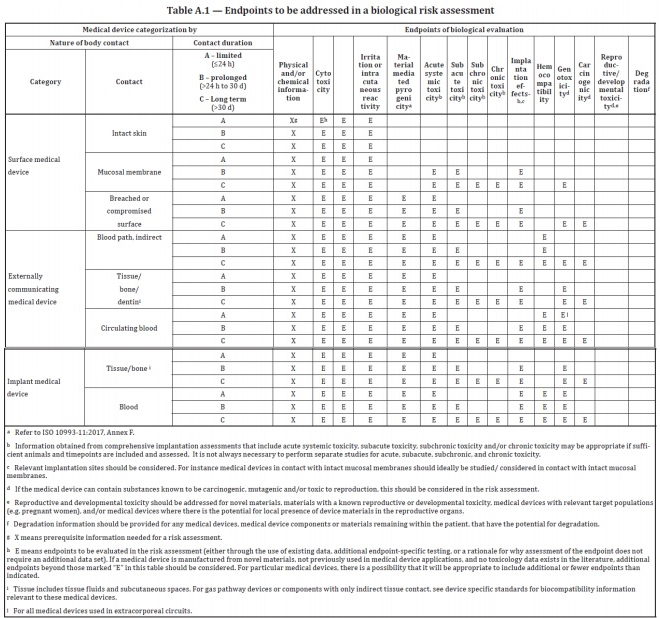
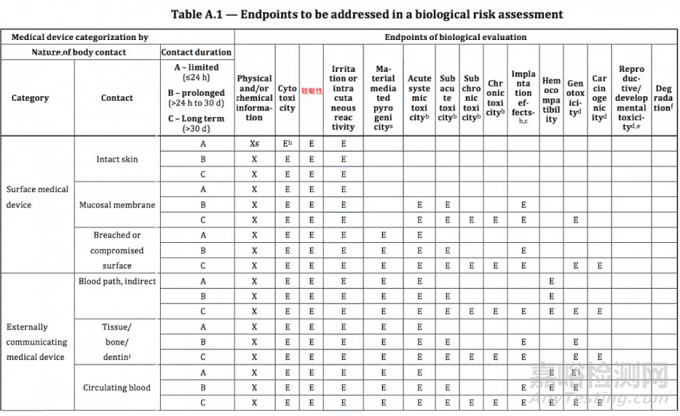
ISO 10993-1:2018(en), Biological evaluation of medical devices — Part 1: Evaluation and testing within a risk management process